MONDAY, 25 NOVEMBER 2013
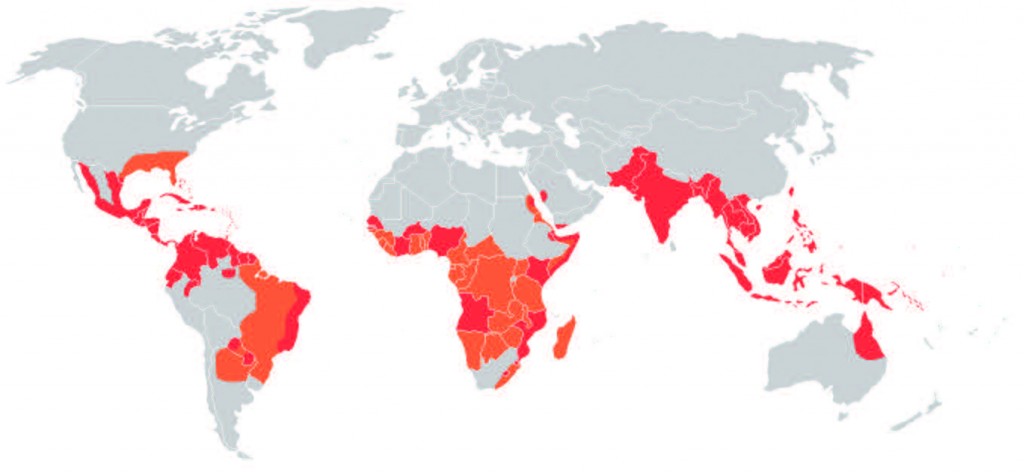
From dengue fever to malaria, mosquitoborne diseases have been a scourge of human communities for centuries. While our current understanding of their causes has greatly improved, halting their spread remains a challenge. Diseasecausing organisms develop drug resistance, overuse of insecticides has severe environmental consequences, and mosquito populations can also become insecticide-resistant. However, there is now a better approach that, if successful, could eliminate mosquito-borne diseases, such as dengue fever. Our unlikely saviour? A bacterium called Wolbachia, which prevents the mosquitoes that carry it from transmitting disease.This is good news for the over 100 countries in which dengue fever is endemic. The World Health Organisation estimates that over 40 per cent of the world’s population is at risk of infection from the dengue virus, which is most commonly transmitted by Aedes aegypti mosquitoes. Controlling dengue is a particularly intractable public health problem: no specific drug treatment is available, no vaccine has yet been developed, and infection with one strain of the virus confers no immunity to other strains. In fact, prior infection increases the future risk of severe complications, such as internal bleeding and organ failure. Until recently, the only ways to combat dengue were to prevent mosquitoes from biting or to eliminate them altogether.
So where does our humble bacterium fit in? Wolbachia’s story begins in 1924, when it was first isolated from another mosquito species, Culex pipiens. Since then, it has been found in many other insects, though surprisingly not in the mosquitoes that carry the dengue virus. Wolbachia is an intracellular symbiont: it lives and reproduces within the cells of its host and is found in a wide range of tissues, such as the brain and gut. Female insects pass Wolbachia on to the next generation through the eggs they lay. In mosquitoes, the bacterium makes transmission even more efficient by killing eggs laid by uninfected females that mated with infected males. This is called ‘cytoplasmic incompatibility’ and ensures that the bacterium quickly colonises entire mosquito populations.
Wolbachia was originally thought to be harmless, perhaps even conferring metabolic benefits on its host. Interest in it as a method of disease control only arose in earnest in 1997, after a novel strain of the bacterium was discovered. This strain was called wMelPop, or popcorn, because of its bizarre effects on the host’s cells. Unlike most other strains, wMelPop, originally found in fruit flies, was highly virulent. It multiplied so rapidly within its host that the infected cells became packed with bacteria, eventually resembling bags of popcorn. This led to cellular degeneration and early death of the infected flies, whose life-spans were half that of uninfected insects. In 2009, wMelPop attracted the attention of dengue researchers after Scott O’Neill’s research group stably introduced the strain into the dengue transmitter Aedes aegypti—which it killed just as quickly as it did the fruit flies, suggesting that this method could help control mosquito-borne disease.
The wMelPop strain turned out to decrease not only the lifespan of Aedes mosquitoes, but also their ability to transmit dengue. In 2009, O’Neill’s group also found that wMelPop-carrying mosquitoes could not be infected with the dengue virus; in other words, Wolbachia was ‘vaccinating’ them. Suddenly, the focus switched from using wMelPop to kill mosquitoes to introducing the dengue virus-preventing bacterium into wild mosquito populations. In 2011, an avirulent strain, wMel, provided a solution: like wMelPop, it could be stably introduced into mosquitoes in the laboratory and could block dengue transmission, but unlike wMelPop it did not harm its host.
This chapter in the Wolbachia story marks the beginning of an international research and fieldwork collaboration: the Eliminate Dengue programme. Its aim is to release wMel-carrying mosquitoes in areas where dengue is endemic. The first field trial, which took place at two locations in northern Australia, has been a resounding success, with wMel colonising at least 90 per cent of the local Aedes populations. This approach has several advantages. First, there is little ecological impact because no insecticides are required and because the mosquito species is not being eliminated from the environment. The second and greatest advantage is the high degree of community involvement at each stage of the trials, which accounts for the global spread of enthusiasm for the project. Releases of wMel are now taking place elsewhere in northern Australia and in Vietnam. Releases are also scheduled for 2014 in Brazil, which has the highest incidence of dengue worldwide, with collaborations planned in China and Indonesia.
However, despite the initial success of the Eliminate Dengue trials, there are several areas that urgently need further research. In the laboratory, wMelPop actually ‘vaccinates’ mosquitoes against the dengue virus more effectively than wMel. However, due to the bacterium virulence, infected mosquitoes may be unable to compete with wild ones under environmental stress, resulting in a less efficient spread of Wolbachia. This is important because Aedes populations in cities and towns often breed in confined, overcrowded conditions, which can lead to increased resource competition. To address this problem, Ary Hoffman’s research group is carrying out detailed studies of the effects of wMelPop infection on Aedes aegypti’s ability to compete with uninfected mosquitoes at different stages of the life cycle.
An alternative approach, which the Hoffmann group published this year, is to breed wMelPop-carrying mosquitoes that are insecticide-resistant. Spraying an area with insecticides just before wMelPop mosquitoes are released could reduce the size of wild populations enough to give the Wolbachia-carrying mosquitoes a competitive advantage. However, besides the ecological and health issues associated with insecticides, this could be counter-productive. We do not yet know if wMelPop’s ability to block dengue attenuates over time; if it does, combining it with insecticide resistance could create a population of resistant, dengue-carrying ‘super-mosquitoes’.
Finally, we still do not know exactly how Wolbachia blocks dengue transmission, though preliminary evidence suggests that both fruit flies and mosquitoes produce higher amounts of protective immune proteins following infection. In the future, a better understanding of the biological mechanism behind Wolbachia-mediated dengue ‘immunity’ could increase the efficiency of disease control.
Will Wolbachia spell the end of dengue fever? It is too soon to tell, but the leaders of Eliminate Dengue remain optimistic. Perhaps we could eventually use this approach to combat a range of mosquito-borne diseases: recent studies have shown that it can also prevent transmission of malaria parasite. In an era of Wolbachia disease control, we might still get bitten, but it would mean irritation rather than life or death.
Joy Thompson is a 1st year PhD student at the Department of Physiology, Development and Neuroscience