MONDAY, 3 FEBRUARY 2014
Let me tell you a story. It begins in Cambridge. It contains hard work, ridicule and, as in all good stories, unexpected triumph. It ends - well you’ll have to wait to find out.In 1905 John Langley, a scientist in Cambridge studying the mechanism of hormone action, became the first person to use the term ‘receptive substance’. His experiments led him to propose that a molecule may cause a biological phenomenon by physically interacting with such a ‘receptive substance’. To many school-aged biology students, this is now obvious. Drugs bind to receptors, surely everyone knows that? However just over a century ago, nobody did.
Around this time German immunologist Paul Ehrlich had similar ideas. His ‘side-chain’ theory, based on the observation that chemicals’ selectivity for certain tissues cannot be accounted for solely by the path they travelled, supposed something on cells’ surfaces must be causing the difference in action.
However the scientific community was far from receptive: Walther Straub, an eminent pharmacologist, described Ehrlich’s postulation of a general theory of binding between drugs and cells as “too far” and “not fruitful”. However Straub wasn’t without motives, having proposed his own counter theory for drug action. This potential-poison theory hypothesised incorrectly that a drug’s effect depended on its concentration difference between the inside and outside of a cell.
In 1908, whilst working in Langley’s lab in Cambridge, Archibald Hill published a mathematically minded paper far ahead of its time. Hill studied the time taken for frog muscle to contract in response to nicotine, showing it fitted very closely the equation expected if there were “a combination between nicotine and some portion of the muscle.” However, Hill never became the renowned pharmacologist he might have, had his ideas been taken more seriously. Still receptor theory was ridiculed, if discussed at all.
By the 1930s a large amount of experimental evidence supporting receptors had accumulated. In 1926 two key papers were published independently, by A.J. Clark and J.H. Gaddum. The pair were sticklers for data, and began quantifying pharmacology, allowing concrete measurements to be made and predictions to be tested. As in so many areas of science, this introduction of rigorous maths proved invaluable to spurring progress. However what really set apart this new research, was the use of two drugs simultaneously. Clark used frog hearts to show that a higher concentration of acetylcholine was required to produce the same effect when given with atropine, the poison from deadly nightshade. However Clark himself failed to conclude that these two compounds might compete for one receptor. Gaddum simultaneously showed a similar phenomenon in rabbit uterus and in 1937 derived the relatively simple equations quantifying the effects of two opposing compounds as they compete for one receptor. This phenomenon is now known as ‘competitive inhibition’.
Jewish German turned Italian citizen Heinz Schild had an illustrious research career, which included studies on antagonists – compounds blocking the effects of hormones or drugs. His work provided further mathematical evidence for the receptor concept through a novel graphical method for determining affinities, however pharmacology at large still appeared distinctly uninterested in the idea that receptors might actually exist. Even many individuals influential in the discovery of receptors were incredibly sceptical of their own work. Raymond Ahlquist helped discover two different adrenaline receptors, but in 1973 said of them that it was “...so presumptuous to believe... receptors really exist. To me they are an abstract concept.”
The scientists looking to isolate these ‘abstract’ receptors and study them in isolation thus faced the challenge of inventing new experimental techniques, but perhaps even more daunting than this was the prospect of overturning half a century’s worth of scientific dogma. Researchers first identified radioactive compounds which bound the receptor. Since radioactivity is easily detected, these allowed analysis of the receptor’s binding characteristics. Though this was a major achievement in itself, researchers still needed to isolate receptors such that they could be studied individually. Receptors are so sparse within living tissue that isolating the chosen beta2-adrenoreceptor, required 100,000 fold purification. This was achieved using novel ‘affinity chromatography’ methods, which involve passing candidate proteins through a column with molecules capable of binding the receptor fixed to its inner surface. Molecules remaining in the column are therefore receptors. These can then be washed through separately from other proteins, giving a pure receptor isolate. Affinity chromatography, along with many other novel identification techniques, meant scientists could finally determine the amino acid sequence making up the receptor.
At the time only two other receptors of any kind were sequenced both capable of detecting light. The two receptors were found to share large proportions of their sequences and several structural features – such as crossing the cell membrane seven times. These features were therefore assumed to relate to their ability to detect light. However, to the surprise of the entire scientific community, the same features were present in the newly sequenced beta-2 adrenoreceptor. No one had anticipated this – why should proteins with such distinct functions be so similar in structure? Within just a few years labs around the world found more and more proteins with this structure and within two decades, the ‘abstract concept’ of receptors had found its way into the mainstream, and the first and major superfamily of receptors, the G Protein Coupled Receptors (GPCRs), were well established.
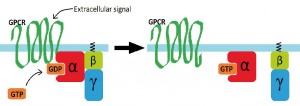
All GPCRs are proteins crossing the outer membrane of a cell seven times. Most have specifically shaped binding pockets, allowing only certain molecules to bind. GPCRs tell the inside of a cell that a signal unable to cross the membrane is present outside the cell. The binding of a molecule to the GPCR causes specific shape changes in the receptor, enabling the GPCR to catalyse the swapping of a GTP molecule for a GDP. This activates the G protein, which splits into two parts; both of which trigger functions within the cell.
Two decades later it is difficult to believe that receptors were ever controversial. They are ubiquitous in biology: allowing us to sense the world around us, use hormones to control our bodily functions, and are targets for countless drugs. GPCRs’ beauty lies in both their simplicity and complexity. The concept – detect a signal, change shape, activate a G protein – is incredibly simple. Yet the structural changes taking place are so complex we still don’t fully understand them. Small variations in the detection step allow this super family to orchestrate our vision, and senses of smell and taste.
Last year Brian Kobilka and Robert Lefkowitz won the Nobel Prize in Chemistry for their work on GPCRs, signalling the end of one chapter of our story - the discovery of these versatile receptors. However the next promises to be both fascinating and revolutionary. There are almost 1000 GPCR varieties in our bodies, including many ‘orphan receptors’ of unknown function. The race is already on to discover their roles and to produce drugs to specifically activate or block certain receptors with known roles in disease.
Having begun our story in Cambridge we have come full circle. Much of the current research into GPCRs is taking place right here right now. We have seen how Langley’s concept gradually gained support through years of often apparently unrelated research, and how this led to the discovery of the GPCR superfamily which holds so much promise. The end of the story? I’m afraid you’ll have to wait for that, the age of receptors is only just beginning.
Toby McMaster is a 2nd year biological natural sciences student at Jesus College.