WEDNESDAY, 3 OCTOBER 2012
Issues of handedness are a common feature of human societies, throughout history it has been used to judge people. Even the terms associated with ‘right’ (dextrous) are perceived as good and correct whilst ‘left’ (sinister) has darker implications. In many societies left-handedness has been punished and conformism to the ‘right’ way has been enforced. However, on the atomic level, handedness becomes even more sinister. Many chemicals also have handedness and for us this handedness can be the difference between life and death.In some chemicals, atoms are joined together in specific conformations to form molecules. These can be small and simple such as water (H2O) – consisting of two hydrogen atoms and one oxygen – or they can be unimaginably large such as DNA, which can be up to two metres long and includes millions of individual atoms of many different types.
Mirror image molecules occur whenever one atom in a molecule is directly attached to four or more different parts of that molecule. These attachments can be organised in two different ways – one a mirror image of the other – and these molecules are known as optical isomers of each other. Optical isomers were first identified in the early 19th century from their differential interactions with certain types of polarised light. Based on how the molecule is arranged, the two forms can be named as the R-isomer (Rectus/Right) or the S-isomer (Sinister/Left) or, in biology, as D and L respectively. Molecules that can have distinct optical isomers are called chiral molecules.
In most cases, optical arrangement doesn’t have any effect and it can be very difficult to tell the difference between the two isomers without very specific tests. However, a chiral molecule will interact with other chiral molecules in specific ways depending on their handedness. This may not seem very important, until you consider that many of the molecules in your body are chiral. Understanding chiral molecules is crucial to understanding illnesses and helps to develop new medical technologies.
Many important molecules in living things are chiral, including sugars, proteins and DNA. In all of these cases one isomer of any chiral molecule is strongly favoured over the other and the same one is favoured in all life on Earth. For example, almost all of the sugars in your body are the R (D)-isomer. Conversely, amino acids, the basic building blocks of proteins are predominantly S (L)-isomers and form left-handed proteins. The processes that release energy from sugars and build amino acids into proteins are highly sensitive to chiral arrangements and are typically unable to make use of the mirror images. The cellular components that drive these processes have evolved to recognise just one optical isomer and cannot process the mirror image because it has a different arrangement that cannot be identified.
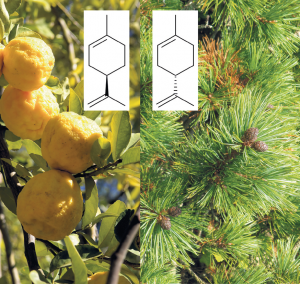
The implications of chirality in the human body vary from the innocent and entertaining to the life threateningly severe. Smells provide some great examples of this. The sense of smell is the result of specialised cells in your nose interacting with molecules in the air that you breathe in. One of these molecules is called limonene. As the name suggests limonene is an important part of the smell of lemons and other citrus fruits, if it is the D-enantiomer. However L-limonene has a fragrance similar to pine trees.
The difference in aroma between D-limonene and L-limonene is the result of how they interact with the smell receptor cells in your nose. Each cell is covered in only one of 900 scent receptor proteins and each protein has a pocket that recognises specific parts of molecules in the air. Since the different parts of D- and L-limonene are arranged differently relative to each other they fit into the pockets of different receptor proteins, activate different cells and so cause you to react differently. You recognise that smell as either citrus fruit or pine.
Whilst limonene is an innocuous example, there are other cases where mistaken identity of isomers has had in tragic and deadly outcomes across the developed world. Many drugs have different enantiomers and generally this is relatively harmless: not so in the case of thalidomide. Thalidomide is a very simple chemical and a chiral molecule. It was developed in the 1950s after being discovered accidentally whilst searching for antibiotics. Originally marketed as a sedative, Thalidomide became known as a wonder drug that could be used to treat a wide range of ailments. One of its most popular applications was as a treatment of morning sickness during pregnancy.
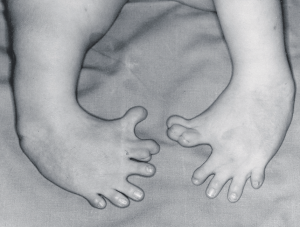
After less than five years on the market Thalidomide was withdrawn from over 50 countries. In just a few years over 10 000 children were born across Europe and Africa with severe developmental defects. These defects, which killed around 40 per cent of the affected children before their first birthday, were shown to be directly due to use of thalidomide by mothers during pregnancy. Although Thalidomide was thoroughly tested for side effects prior to release and was found to have no obvious toxic effects directly on the patients treated, it was never tested on pregnant women.
The birth defects caused by Thalidomide were linked to how much of the drug was taken and when during the pregnancy it was used. Defects could be minor and relatively superficial including loss of gain of fingers and toes, although more commonly babies had completely underdeveloped limbs, known as phocomelia. In the most severe cases babies had fatal defects such as restricted lung growth, underdeveloped hearts, nerve dysfunction or intestinal defects.
In the case of Thalidomide the isomers live up to their names; the R (right)-enantiomer has health benefits, whereas the S (sinister)-enantiomer is responsible for causing birth defects. Sadly, it is not possible to have the beneficial drug without having its evil twin. Even though R-Thalidomide can be made without the S-isomer and given to patients, once inside the human body it is possible for S-thalidomide to be made from R-thalidomide in a process called racemisation. Despite this thalidomide continues to prove itself a wonder drug with many new applications still being uncovered. After all, thalidomide is still harmless for use by anyone who is not currently pregnant.
In the modern age handedness may seem like a trivial issue – we no longer beat our children for showing left-handed tendencies – but when it comes to molecules in chemical reactions a left-handed molecule and its right-handed counterpart, in the right context, may still be polar opposites. One chemical can either give life, or it can take it away. Of course in most cases the difference between optical isomers is much less severe and is simply an amusing scientific curiosity: the difference between lemon and pine.
Jonathan Lawson is a 2nd year PhD student in the Department of Genetics