THURSDAY, 20 MAY 2021
From beautiful gemstones in underground caverns to salt-encrusted rocks by the ocean, crystal structures are found throughout the material world. Not only are they beautiful but they are also highly functional. By understanding how crystals grow, we developed exciting technologies of the Digital Age, paved the way for safer planes and even explained the magic of snowflakes.So what makes up a crystal?
We start at the atomic level.
Although the term “crystals” brings to mind colourful, faceted gems often used in jewellery, many solids actually form crystalline structures. A crystal structure simply refers to the ordered and periodic arrangement of atoms (or molecules) within a material.
Imagine, for instance, a cube with an atom placed at each corner. By repeating this cube in three dimensional space (x, y, and z directions), a crystal is formed. If instead of a cube, the atoms arrange into a rectangular prism or a hexagonal prism, a different crystal structure with different symmetries and physical properties will form.
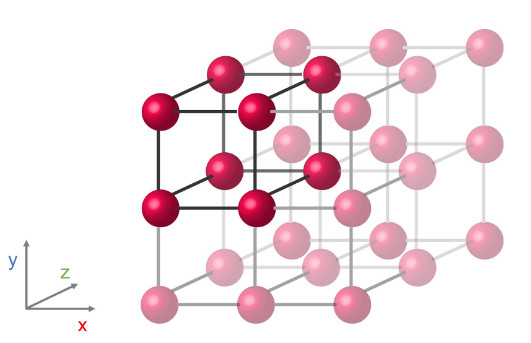 A simple cubic crystal structure with an atom at each corner of a cube.
A simple cubic crystal structure with an atom at each corner of a cube.These atomic positionings are determined by the conditions that lead to crystallization.
How are crystals formed?
Examples of crystal formation are all around us. A simple one is cooling molten metal into a crystalline solid state: in the liquid state, the metal atoms have plenty of kinetic energy to move around and the probability of atoms forming a cluster is very low - imagine energetic children running around in a classroom. As the temperature decreases, the atoms lose their kinetic energy, becoming increasingly likely to cluster. When the temperature drops below the freezing point, a solid crystalline state becomes more favorable than the liquid state - now imagine tired children who would rather sit at their desks arranged in neat rows. Any cluster of atoms that forms then has the potential to arrange into a tiny crystal called a nucleus. If the surface area (total energy cost of nucleation) to volume (total energy gained) ratio is less than a certain value, the nucleus can be stabilized. The atoms at the edges provide a specific orientation for neighbouring atoms in the liquid phase to align and attach, thus growing the crystal.
Another example is the formation of salt crystals from solution. As the water evaporates, the concentration of dissolved salt ions increases, until the saturation point is reached. Beyond this, the solid crystalline state is energetically more favorable than remaining in solution, and clusters of salt ions have the potential to nucleate. Salt crystals form cubic arrangements of atoms, and close examination of any crusty rock at the beach will usually reveal small white crystal cubes! Similarly, stalactites and stalagmites are formed in caverns when limestone crystallizes from drops of concentrated solution.
Atmospheric water freezing into snowflakes also follows the nucleation-and-growth theory. Below a certain temperature, the saturation limit of water vapor in air is exceeded, and the preferred state of water is a solid, meaning that ice crystals can nucleate and grow. Due to the arrangement of water molecules into a hexagonal array, beautiful six-sided snowflakes are formed.
Furthermore, the same material can form different crystal structures depending on the crystallisation conditions. These differences in atomic positionings lead to different physical properties. In steel, one crystal structure causes it to become extremely hard and brittle, while another arrangement of the same atoms results in a more pliable steel. The reason Japanese samurai swords were so incredible is because the blade edge was made of brittle crystalline steel for cutting, while the body had atoms arranged in the more flexible crystal structures to absorb shock. In order to achieve this, swordsmiths apply a different cooling rate to the edge compared to the spine of the sword.
As we can see, crystallization dictates the atomic positionings which in turn dictates the material properties. Specific crystals with specific electronic, thermal, or physical behaviours can be synthesized and used to improve our lives.
What are crystals used for?
Without crystals, we would not have computers, planes, electric cars, many pharmaceutical drugs, or even vaccines. It doesn’t take a crystal ball (which are sometimes just glass balls) to see that our lives would be drastically different without understanding and harnessing the magic of crystals.
Silicon single crystals serve as a semiconducting substrate onto which microelectronic devices are fabricated, such as the transistors that make up a computer chip. Since polycrystalline silicon have poor electronic properties, developing methods to grow single crystal silicon efficiently and economically was a major factor in launching the world into the Digital Age (also known as the Silicon Age)! Other single crystal semiconductors with precise electronic properties are also produced at large scales for the LED lights, solar panels and sensors that are ubiquitous today.
Lithium-ion batteries that power most of our portable devices and electric vehicles are also reliant on the crystalline materials. The energy provided by a battery comes from lithium ions entering a crystalline host structure. Changes in the crystal structure can affect the amount of energy delivered and the ease of charging or discharging the battery, ultimately affecting how long your battery can last.
Another class of interesting crystals are piezoelectric materials. These materials demonstrate a change in electrical conductivity when the crystal structure is elongated or compressed in a particular direction. Ultrasound transducers, used for non-invasive medical imaging like pregnancy monitoring, employ piezoelectric crystals to detect the returning ultrasound echos. The soundwaves cause a slight deformation in the detector’s crystal structure resulting in electrical signals that are converted to an image.
Thermal properties can be improved by crystals too. Single crystals of nickel alloys are grown and used as jet-engine turbine blades because their thermal stability and mechanical strength exceeds that of steel, which is prone to cracking during operation. Without these single crystals, catastrophic failure of turbine engines would make planes very unsafe!
Crystallization can even occur in molecules that assemble into periodic arrays. Biological materials such as proteins and DNA can pack into ordered layers that form crystals up to hundreds of micrometres in size. The double helix structure of DNA and many important protein structures were discovered by analysing their crystals.
Ongoing research in crystals is found across all disciplines. In chemistry, understanding and controlling the crystal structures in battery materials can lead to better performance in phones, laptops and electric vehicles. While in biology and pharmacology, research in protein crystallization is important for the development of novel vaccines and drugs. A recent example of this was the rush to solve coronavirus protein structures.
Our world consists of and is built upon crystal structures. Many natural wonders as well as many of our technological advances are a direct result of crystal magic. Who knows what weird and wonderful crystals we will discover or formulate in the future...
Evelyna Wang is a 3rd year PhD student in Chemistry at Girton College. Artwork by Jocelyn Tang.
